Conventional in-ground wastewater disposal systems cannot be counted on to adequately protect groundwater. It surprises many people to learn that even a properly functioning septic system built to code is designed to introduce pollutants into the soil and—ultimately—the groundwater. Worse, a large number of the roughly 22 million in-ground wastewater systems in this country are either inadequately designed or failing.
We can do a lot better. Interest is rapidly growing in a number of alternatives to conventional in-ground wastewater disposal systems. These alternatives include on-site composting of toilet wastes with graywater separation, constructed wetlands, and sand filters. We are just beginning what is likely to be a wholesale shift in the way we design on-site wastewater disposal systems. This article takes a look at conventional septic systems and describes three environmentally preferable alternatives. Municipal sewage treatment is not addressed.
Conventional Septic Systems
To understand why conventional in-ground treatment and disposal systems do not effectively treat wastewater, you have to know something about how they work and what they’re treating.
Wastewater includes a number of significant contaminants. The most commonly measured components of wastewater are biochemical oxygen demand (BOD), suspended solids, nitrogen, phosphorous, and coliform bacteria. BOD is the amount of organic matter present based on the amount of oxygen required by microorganisms to decompose it. BOD
5 is a measure of that demand over a five-day period. Fecal coliform are bacteria that live in the human digestive tract; while not dangerous themselves, they serve as indicators of pathogens that might be present.
Wastewater contaminants can be measured by concentration in wastewater (milligrams per liter, or numbers per liter), or by how much is produced per person in the building (grams per capita per day). Typical contaminant levels in residential wastewater are shown in Table 1.
Surprisingly,
graywater (usually defined as all wastewater except that from toilets) typically introduces more BOD5 and almost as much suspended solids as blackwater—even if garbage disposal output is not included in the graywater component. Studies of microorganisms in blackwater and graywater have produced highly variable results. Results of three studies of bacteria in graywater are shown in Table 2.
While graywater clearly contains high levels of organic matter, some claim that the organic matter (BOD) in graywater is different from that in blackwater. In a report
Using Greywater: A Basic Planning Manual for the Processing of Greywater, Clivus Multrum, Inc. presents evidence from Europe that the organic matter in graywater breaks down much faster (almost 65% per day) because it is more readily available to microorganisms.
In a conventional septic system, wastewater flows from the building into a septic tank where solids settle out. The bacteria in the septic tank’s anaerobic environment carry out some biological decomposition, but most of the contaminant removal occurs when the material that settles to the bottom of the tank (septage) is pumped out. Based on limited studies presented in the EPA
On-Site Wastewater Treatment and Disposal Systems Manual, BOD5 concentrations are reduced 30% to 70% in septic tanks, suspended solids concentrations are reduced 60% to 90%, total nitrogen levels are reduced 30% to 40%, and phosphorous levels are reduced about 15%.
Effluent passes from the septic tank to a drainfield (sometimes called an absorption field or leaching field). There the effluent seeps into the ground where aerobic soil bacteria break down most of the organic matter, including pathogens. If the percolation rate is too great, however, or either air or soil bacteria are limited, BOD may not be adequately removed. If high-BOD effluent makes its way to surface water (streams, ponds, etc.), it can use up oxygen in those bodies of water. With inadequate oxygen, fish die and anaerobic bacteria take over, resulting in stagnant, smelly conditions. Over time, an anaerobic biomat may also form in the drainfield, restricting effluent flow and leading to system failure.
Even a properly functioning drainfield does not remove most nutrients (nitrogen and phosphorus). These are among the most harmful pollutants in wastewater because if they reach surface waters (streams, ponds, etc.), they fertilize algae, whose growth can cause eutrophication. In this process, prolific algae growth covers the surface of the water, blocking sunlight penetration into the water. Without sunlight, underwater plants cannot carry out photosynthesis. As the plants die and decay, oxygen in the water is used up, killing fish and most other freshwater life. This result is similar to that described above with BOD pollution, but its effects are usually even more severe.
Along with contaminating bodies of water, high nitrogen levels in drinking water can cause problems. According to Sherwood Reed, P.E., of Norwich, Vermont, a leading authority and EPA consultant on wastewater disposal, “there are groundwater resources all over the country that are contaminated with nitrate.” Reed considers it a virtual certainty that in any village center in New England where in-ground septic systems are used, the nitrate level in groundwater will exceed federal drinking water standards (10 mg/l). For infants, nitrate poisoning is a significant risk, causing methemoglobinemia (blue baby syndrome), and some people consider high nitrate levels to be carcinogenic.
The other problem with conventional on-site wastewater systems is septage disposal. Standard practice today is to pump it out and take it to a municipal sewage treatment facility. Even though sewage treatment plants often do a pretty good job at removing pollutants from water, large quantities of sludge are produced that require landfilling, surface application to land, or incineration. Contaminants other than those from human waste and household cleaning products end up in municipal sewage sludge, making it unsuitable for many applications.
Given these problems with conventional on-site wastewater treatment, environmentally concerned designers and builders should consider alternatives. Three alternatives to conventional in-ground wastewater treatment and disposal are discussed below. Each provides significant environmental advantages over conventional systems.
Composting Toilets
A properly managed composting toilet offers the most environmentally attractive option for treating and disposing of human waste. The basic strategy is to keep human wastes totally separate from graywater. As soon as you mix human excrement and urine with water, according to Abby Rockefeller, president of Clivus Multrum, Inc., treatment becomes more complex and expensive. By biologically breaking down the wastes, nutrients can be returned to the land as fertilizer. Perhaps most important, there is no septage that needs to be treated and/or disposed of off-site.
There are several different types of composting toilets (referred to as biological toilets by some, since true, high-temperature composting conditions are not reached). The most familiar composting toilet is the Clivus Multrum, introduced in the United States in the early 1970s. It has a large holding tank situated directly beneath the toilet—usually in the basement. Human wastes mixed with kitchen compost and other high-carbon material, such as peat moss, accumulate in the tank where aerobic bacteria, worms, and other organisms break down the wastes over a period of months. Humus is removed at the bottom end after a 6- to 12-month retention time. Most liquids evaporate and exit through the ventilation stack, though a recent design change provides for separate collection of liquid (compost tea), which the company describes as an odor-free, high-nutrient fertilizer. A residential-sized Clivus Multrum composting toilet costs $2,000 to $3,000 (plus shipping and installation), depending on size and features.


A second type of composting toilet is smaller and more compact.
Human wastes and organic matter (toilet paper and peat moss) are mixed in the holding tank. Ventilation provides oxygen for aerobic bacteria, liquids evaporate and exit through the vent stack, heat is provided by compost action and a supplemental heater, and periodic mixing is done automatically or with a hand crank. Humus is removed via a special drawer every few months as required. Some of these systems can be used with ultra-low-flush (1 pint per flush) toilets. Sun-Mar, the leading producer of these toilets, offers models ranging in price from $1,000 to $1,300.
A third type of composting toilet is more high-tech. The AlasCan composting toilet and graywater treatment system was introduced in the late 1980s for use in permafrost regions and is more automated and “lifestyle independent” than other composting toilets—but also more complex and expensive. An ultra-low-flush toilet is used to deliver wastes to a tank located in the basement. Kitchen food wastes are delivered to the composting toilet after shredding in a garbage disposal, and additional wood chips or coarse sawdust is added. In the heavily insulated tank, bacteria, worms, and other organisms break down organic matter. There is a water recirculation system that collects water at the bottom of the tank and returns it to the organic matter. Automatic agitators mix and aerate the material, and an air-to-air heat exchanger exhausts air and delivers preheated fresh air. Total system cost for an AlasCan toilet and graywater system is $9,000 to $10,000, plus shipping and installation.
Composting toilets effectively deal with human wastes but not graywater. Both Clivus and AlasCan offer systems for treating graywater. The AlasCan system is fully integrated and even provides for delivery of sludge from the graywater settling tank into the composting chamber.
There is growing interest in graywater separation and use, particularly in drought-prone areas of the country. With fairly simple treatment, proponents argue, graywater can safely be used for landscape irrigation and toilet flushing. The National Association of Plumbing-Heating-Cooling Contractors in Falls Church, Virginia, one of the nation’s four model-plumbing-code-writing associations, is heavily involved in advancing graywater separation and use. Reuse of graywater will be addressed in greater detail in a future article.
Issues of Concern with Composting Toilets
1.
Graywater treatment/disposal is still required. Most codes around the country currently require that graywater be treated just the same as blackwater (i.e., same size septic tank and drainfield), so there are no savings to pay for the composting toilet. Revisions to plumbing codes are gradually changing this situation.
2.
Performance depends on the operator. Unlike conventional flush toilets, composting toilets require proper management to function as designed. Organic matter needs to be added, some systems require periodic mixing, and you have to be careful about what goes in.
3.
Potential for overfertilizing. The humus and compost tea from composting toilets have very high nutrient concentrations and must be carefully applied to prevent nutrient build-up. They are most appropriately used where nutrients will be removed through harvesting—not in natural ecosystems.
4.
Concern about pathogens or toxins in composted waste. Composting toilets do not reach high enough composting temperatures to guarantee destruction of all pathogens. Experts recommend against direct application to vegetable crops, or at least root crops. The optimal strategy may be to apply this fertilizer on cover crops used in rotation, on tree crops, or on nonfood crops (nursery stock, ornamentals, etc.).
5.
Unconventional and requires homeowner involvement. As a society, we don’t like to think about our excreta, and the thought of keeping it in our houses just doesn’t feel right to most people. More than any other reason this is why composting toilets have not caught on more widely—and are unlikely to do so. Composting toilets will probably find much greater use in public buildings, such as park facilities.
6.
Energy use. Composting toilets use energy to operate ventilation fans and, in some cases, heaters, agitators, and other mechanical equipment. Over the course of a year, a continually operating fan—even a small one—uses a lot of electricity. Ventilation will also increase heating and cooling loads.
Constructed wetlands are the newest, and in many ways most exciting, alternative to conventional on-site wastewater treatment. They are exciting because they so closely mimic natural systems in their operation, harboring diverse, complex ecosystems. By constructing artificial wetlands, we also learn more about the value of
natural wetlands—and why we need to protect them.
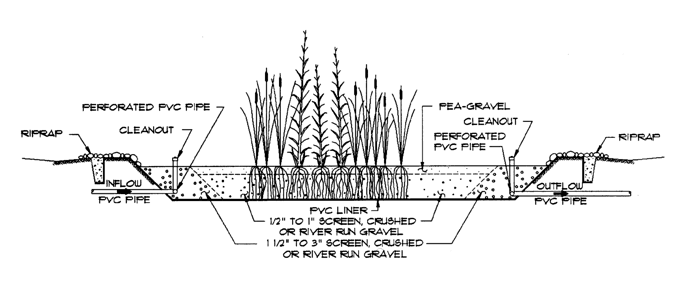
The idea of building artificial wetlands specifically for wastewater treatment dates back to the late 1960s in Germany, but did not gain significant attention until the 1980s. They are used today both for municipal sewage treatment and for on-site treatment. In the U.S., the first work with constructed wetlands wastewater treatment was done in the early 1980s.
Some of the earliest work was done by NASA at the Stennis Space Center in Mississippi, and by Dr. B. C. Wolverston in Picayune, Mississippi. They termed this system a
microbial rock plant filter. Constructed wetlands expert Sherwood Reed estimates that there are 500 constructed wetland systems in operation in the U.S. today, one-half to two-thirds of which are on-site treatment systems, the rest municipal.
Constructed wetland designs are evolving at a rapid pace based on on-going research. While some constructed wetlands have exposed surface water,
subsurface-flow wetlands are more practical for on-site wastewater treatment. Because water is never at the ground surface, mosquito breeding, odor, and risk of human contact with effluent are effectively eliminated. A typical system is shown in the illustration on page 15. The wetland is one to three feet deep and built with an impervious bottom and sides—usually a plastic pond liner or poured concrete. Effluent from the septic tank is introduced on one side of the wetland through diffusing pipes, and the liquid flows through a layer of clean, washed gravel (1/2" to 1" diameter) in which emergent wetland plants are growing. The most common plants for constructed wetlands are common reed (Phragmites), cattail (Typha), pickerelweed (Pontederia), arrowhead (Sagittaria), and bullrush (Scirpus), but others can be used.
While early constructed wetlands tended to be quite deep, recent research has shown that most root growth and biological activity is concentrated in the top foot. Wetland plants, because they are adapted to growing in stagnant water, have developed mechanisms to deliver oxygen (required for respiration) to their roots. Bacteria living in association with plant roots do most of the work breaking down pollutants and organic matter. A total hydraulic retention time of one to seven days is typical, though longer retention times during colder winter months are often desirable. Water depth in the gravel is typically controlled by outlet valves .
Nitrogen removal in wetlands is accomplished through several different biochemical steps.
Ammonification is the conversion of organic nitrogen-containing compounds such as amino acids into ammonia (NH3).
Nitrification, which requires aerobic bacteria, is the conversion of ammonia into nitrate (NO3); this can occur near the wetland plant roots where oxygen is pumped down into the wetland.
Denitrification is an anaerobic process in which bacteria convert nitrate into atmospheric nitrogen (N2)—a harmless component of our atmosphere. Finally, plants can take up either ammonia or nitrate and incorporate it into their tissue. For nitrogen to be removed from constructed wetlands by this last process, however, the plants must be harvested. Even in wetlands where plants are harvested, studies have shown that less than 10% of the nitrogen goes into plant tissue; most goes into the nitrification-denitrification reactions of bacteria.
With on-site wastewater treatment systems, the treated effluent is usually collected at the outlet side of the wetland and then delivered (by gravity or pump) to the drainfield. In other words, the entire constructed wetland is an intermediate step between the septic tank and the drainfield. The advantage is that the effluent reaching the drainfield is much cleaner and less likely to pollute the groundwater. Because the effluent from a constructed wetland is so much cleaner than effluent from a septic tank, reasonable arguments can be made for downsizing the drainfield, the savings from which can pay for some of the cost of the wetland. Whether or not the drainfield can be downsized depends on the wastewater flow (hydraulic loading rate), soil characteristics, and local codes.
As for how effective constructed wetlands are at removing pollutants from wastewater, we still do not have good answers. The July 1993 EPA report,
Subsurface Flow Constructed Wetlands for Wastewater Treatment: A Technology Assessment, provides the most complete overall picture of performance. BOD removal is excellent, with effluent levels well below the 20 mg/l reference level commonly used in assessing wastewater effluent quality, and often well below 10 mg/l. Interestingly, much of the BOD measured in constructed wetland effluent may come from the wetland itself, not the
wastewater.
Total suspended solids removal is also very good, with effluent levels typically less than 10 mg/l. Nitrogen removal is more variable. Most organic nitrogen is converted to ammonia. Nitrification and denitrification reactions depend on the bacterial ecosystems in the wetlands. A lot of attention is going into design of wetlands specifically to achieve good nitrogen removal—such as shallower bed design and strategies to achieve deeper root penetration.
Constructed wetlands are most widely used in the southern part of the U.S. (especially Kentucky, Louisiana, and Mississippi), but their viability in more northern climates is being increasingly demonstrated. Sherwood Reed has recently designed a residential-scale system for Burlington, Vermont, and Southwest Wetlands Group, Inc., of Santa Fe, New Mexico, has designed systems in Wyoming, Montana, Michigan, Rhode Island, Vermont, and Massachusetts. For northern climates, somewhat deeper systems are often needed to prevent freezing. It may also be necessary to partition the wetland so that flow in one section can be increased in the winter to prevent freezing, a strategy Reed used in his Vermont system.
Costs of constructed wetlands are highly variable and site-specific. In 16 residential-sized constructed wetlands in an Alabama demonstration program financed by the Tennessee Valley Authority (TVA), installed costs, including septic tanks, ranged from $2,550 to $10,020, with an average of $6,120, of which $2,070 was for labor (design fees were not included in the costs). Hydraulic loading rates for these systems ranged from 83 gallons per day (gpd) to 600 gpd, and constructed wetlands ranged in area from 108 ft2 to 784 ft2.
Issues of Concern with Constructed Wetlands
1.
Designs are not standardized. Except for a TVA design manual (see EBN
Vol. 3, No. 1), there are few specific design guidelines, let alone off-the-shelf constructed wetland designs available. At present, each system must be custom designed.
2.
Freezing potential in northern climates. If wastewater flow rates are low, the constructed wetland shallow, or snow cover absent, the system could freeze in very cold conditions. There is currently very little experience with these systems in northern climates.
3.
Reduced performance in cold weather. Biological processes slow down as temperatures drop. In northern climates this could result in higher pollution concentrations being introduced to the drainfield during the winter months.
4.
Operation and maintenance required. Constructed wetlands are complex ecosystems. Climate and operating conditions may change the performance of the system (drought, heavy rains, changing wastewater flows, etc.).
5.
Systems not recognized in codes. Even though good arguments can be made for permitting, smaller drainfields when effluent flows through constructed wetlands, this is not yet accepted in most areas, making economic justification difficult.
6.
New technology. There is still very little operating and performance experience with constructed wetlands.
7.
Septage still a problem. Septic tanks are still required with constructed wetlands, and the residual solids from septic tanks have to be treated and disposed of.
Sand Filters
Sand filters have been around for almost a century, but until recently have seldom been used as a component of in-ground wastewater treatment systems. They function somewhat like constructed wetlands, but are smaller and provide a more controlled bacterial environment. The most common type of sand filter is installed between the septic tank and the drainfield (see illustration). Effluent from the septic tank periodically doses the top surface of the sand filter and percolates through the sand (usually about 24" deep) where aerobic bacteria break down organic matter and nitrify the ammonia. Beneath the sand layer is a gravel layer where effluent is collected and delivered by gravity or a pump to the drainfield. A liner on the bottom and sides of the sand filter contains the effluent. The entire filter is covered with filter fabric and a thin layer of soil planted with grass. Because the septic tank effluent is delivered in doses, the system is called an intermittent sand filter. In some designs the effluent is recirculated back through the sand filter several times.
More than 10,000 intermittent sand filters have been installed in the states of Oregon, Washington, and California since 1976. Orenco Systems, Inc., of Roseburg, Oregon, is the leading designer and supplier of sand filter components. Most installed systems are 360 ft2 (10' x 36' or 18' x 20'), the “one-size-fits-all” standard for the state of Oregon. Experimental systems are being installed in Washington, Alaska, Massachusetts, and British Columbia with filters as small as 100 ft2.
Sand filters are very effective at removing BOD, suspended solids, and fecal coliform, and they convert most ammonia to nitrate. Because of the effluent purity from sand filters, the drainfield can be significantly downsized, according to proponents. Biomats do not form in the drainfields, and absorption rates are higher than with conventional septic tank effluent. The state of Oregon now permits a 70% reduction in drainfield area with the standardized “Oregon Intermittent Sand Filter,” and Washington permits a 50% reduction. Orenco Systems has been testing a very shallow drainfield design with no imported rock fill.
The installed cost of a sand filter and shallow drainfield (including septic tank) is about $6,000, according to Orenco. The extra cost of adding a treatment step between the septic tank and the drainfield should be offset by the greatly reduced cost of the drainfield, though downsizing the drainfield is not yet acceptable in most states. In situations where this approach might obviate the need for a raised-bed drainfield, significant savings could be achieved.
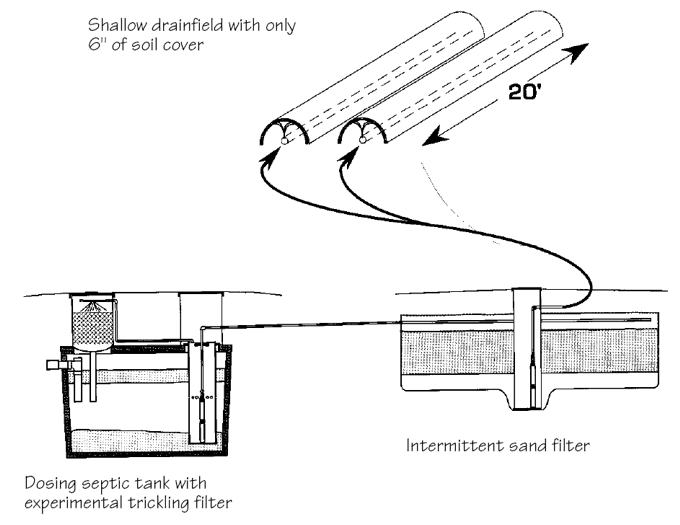
Along with pioneering work with intermittent sand filters, Orenco has been testing a trickling filter that is integrated with the septic tank.
The system was developed specifically to reduce nitrogen levels in effluent from intermittent sand filters, and the company expects to introduce it this spring. With this system, effluent in the septic tank is continually pumped into a small trickle filter located above the septic tank (see illustration). The effluent trickles through the filter, and bacteria remove nitrogen through nitrification and denitrification processes. These two nitrogen-removal reactions can occur because the trickling filter operates aerobically, while the septic tank itself is anaerobic. Cost of the trickling filter add-on to a septic tank is expected to be in the range of $500 to $600.
Issues of Concern
with Sand Filters
1.
How small can intermittent sand filters be made without sacrificing performance? If they can be made small enough to be delivered by truck and filled with sand on-site, cost may come down substantially.
2.
Intermittent sand filters, recirculating sand filters, and trickling filters are mechanically complex. What will the long-term durability of components be? How repairable will they be?
3.
Septic tank quality. To function properly, sand filters require watertight septic tanks, and experience is showing that in many parts of the country, tanks are not watertight. Significant improvements in septic tank manufacturing and quality control are needed.
4.
Energy requirements. Although passive siphon-dosing systems can be designed in some situations, most intermittent sand filters and all recirculating sand filters require pumps. Electrical consumption may be significant over time.
5.
Septage still a problem. Septic tanks are still required with sand filters, and the residual solids from septic tanks have to be treated and disposed of.
A number of factors are pushing us in the direction of improved on-site wastewater treatment systems. First, there are high levels of nitrate pollution from septic systems in more populated village centers throughout rural America. Second, we are learning more about the biochemistry of wastewater treatment and can design artificial ecosystems to remove selected pollutants. Third, funding for improvements to municipal wastewater treatment plants reverted from the federal government to state and local governments during the 1980s; more and more communities cannot afford proper municipal sewage treatment and are looking for on-site alternatives.
This article has reviewed several wastewater treatment alternatives, but by no means all of the available options. Exciting work is being done by Dr. John Todd and his consulting firm, Living Technologies, in Burlington, Vermont, on Solar Aquatic Systems. These systems treat wastewater as it cascades through containers of water rich with aquatic life. Another approach is to use mechanical aeration chambers to support aerobic bacteria. Though energy-intensive and mechanically complex, this system provides a reasonable alternative to conventional in-ground septic systems in some situations.
In considering our wastewater disposal problems, a whole new paradigm emerges: the idea of looking at wastewater as a usable resource instead of simply waste. From an environmental standpoint, composting toilets and separate treatment and use of graywater probably offer the best option. Useful fertilizer is produced, water use is dramatically reduced because conventional flush toilets are eliminated, and there is no residual septage to treat or dispose of. Unfortunately, if experience to date with composting toilets is any indication, we are unlikely to see wide acceptance of these systems.
For most clients, it will continue to make the most sense to keep the graywater and blackwater combined and use either a constructed wetland or sand filter to provide treatment. We still have a lot to learn about the design and operation of these alternative systems, but enough systems have now been installed and are operating successfully that we can begin using them more widely. As we learn more, it is likely that designs will be simplified, standardization will occur, and costs will come down.
A very exciting aspect of these alternative strategies is that we can begin integrating wastewater treatment and disposal systems into the landscaping around buildings—a concept that opens up all sorts of potential. We can treat the wastewater enough to use it safely for irrigation. Constructed wetlands can become valuable assets to the landscaping around buildings—especially if we call them “flower beds.” It is quite conceivable that within a few years it will be landscaping professionals who deal with wastewater treatment, not sanitary engineers.
– Alex Wilson
For more information:
AlasCan, Inc.
3400 International Way
Fairbanks, AK 99701
907-452-5257
Canada Mortgage and Housing Corporation (CHMC)
700 Montreal Road
Ottawa, ON K1A 0P7 Canada
613-748-2000
Clivus Multrum, Inc.
104 Mount Auburn Street
Cambridge, MA 02138
800-425-4887, 617-491-0053 (fax)
Living Technologies
431 Pine Street
Burlington, VT 05401
802-865-4460, 802-865-4438 (fax)
National Association of Plumbing-Heating-Cooling Contractors
PO Box 6808
Falls Church, VA 22046
800-533-7694, 703-237-7442 (fax)
National Small Flows Clearinghouse
West Virginia University
PO Box 6064
Morgantown, WV 26506-6064
800/624-8301, 304/293-3161 (fax)
Orenco Systems, Inc.
2826 Colonial Road
Roseburg, OR 97470
503-673-0165, 503-673-1126 (fax)
Southwest Wetlands Group
PO Box 9280
Santa Fe, NM 87504
505/988-7453
Sun-Mar Corp.
5035 N. Service Road, C2
Burlington, ON L7L 5V2 Canada
416/332-1314, 416/332-1315 (fax)
Tennessee Valley Authority
Water Management Resources Group
1101 Market Street
Chattanooga, TN 37402
615-751-7314, 615-751-7479 (fax)
US EPA
ORD Research Information
26 West Martin Luther King Drive
Cincinnati, OH 45268-1072
513-569-7562
(1994, March 1). On-site Wastewater Treatment: Alternatives Offer Better Groundwater Protection. Retrieved from https://www.buildinggreen.com/departments/feature